
Pre-emptive Dextromethorphan Compared with Midazolam for Premedication in Children
Diana Butkovic*, MD, MSC, Sandra Kralik*, MD, Martina Matolic*, MD, Mirko Zganjer** MD, MSC, Sanja Toljan*, MD, Jasminka Jakobovic*, MD, Ljerka Radesic*, MD, MSC
Acknowledgements
We would like to thank Diana Butkovic, MD, MSC and all Authors for their interesting study and for contribution gived to our Journal.
(Dario Galante, MD, Scientific Manager of APN)
SUMMARY STATEMENT
The aim of this study was to investigate the possible sedative and pre-emptive action of dextromethorphan on postoperative analgesic requirements in children when used for premedication compared with midazolam.
ABBREVIATED TITLE
Dextromethorphan or midazolam for premedication in children
KEYWORDS
pre-emptive, dextromethorphan, midazolam, premedication, children
ABSTRACT
Background: Dextromethorphan is N-methyl-D-aspartate (NMDA) receptor antagonist having mild analgesic and sedative effects. The aim of this study was to test the hypothesis that dextromethorphan, having sedative effects, can be used for premedication in children in comparison with midazolam. Because of its pre-emptive analgesic properties there may be a decrease in the level of postoperative pain and in the amount of analgesic consumption. Methods: Seventy ASA I and II children scheduled for hernia and hypospadias repair, circumcision or orchydopexy were randomized in two groups: the first group (n=35) received dextromethorphan 0.5 mgkg -1 orally 45 min before the induction of anaesthesia, the second received (n=35) midazolam 0.5 mgkg -1 per os. The anaesthesia procedure was standardized. University of Michigan Sedation Scale ( UMSS) scores were measured before the operation and two hours later, pain was measured with visual analogue scale (VAS) and faces scale, according to patients' age at 2, 6 and 12 hours postoperatively. Postoperative analgesic requirements, total dose of rescue analgesic (Diclofenac supp 1 mgkg -1 ) and dextromethorphan side effects were noted. Results: There were no significant differences between the groups in terms of age, weight, gender, ASA physical status, the type and duration of operations . According to the sedation score, preoperative sedation was less with dextromethorphan, but did not reach statistical significance (P=0.531) while postoperative level of sedation was significantly higher in the midazolam group (P <0.002). Comparing the VAS between the two groups at 2, 6 and 12 hours postoperatively we have found that the difference was insignificant at 2 and 6 hours (P =0.635 and P =0.447) while the VAS scores at 12 hours were significantly lower in the dextrometorphan group (P <0.002), but being 2.1 versus 2.4 probably having no clinical relevance. The number of rescue analgesia showed no significant difference between the two groups (0.11±0.3 in the dextromethorphan group and 0.13±0.5 in the midazolam group; P =0.451). Conclusions: Dextromethorphan administered in a dose of 0.5 mg/kg per os has less sedative effects than midazolam. Postoperative level of sedation is higher in the midazolam group, what is not useful for one-day surgery. The level of postoperative pain measured by VAS and faces scale and the postoperative analgesia requirements seemed equal between the groups in the early postoperative period so there was no pre-emptive effect of dextromethorphan noted.
INTRODUCTION:
Dextromethorphan is a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist known to have preemptive analgesic actions, i.e. it prevents the central sensitization in the dorsal horn neurones if given before the painful stimuli (1-7). Dextromethorphan, as a dextrorotatory morphinan without affinity for opioid receptors, has been commonly used as an antitussive medication for more than 40 years with a wide margin of safety. It has a mild sedative effect (6-15). Dextromethorphan is rapidly metabolised in the liver in its demethylated derivative dextrorphan and its weak side effects could be attributed to this metabolite acting on phencyclidine receptors (9). In many studies in adults and children, the perioperative administration of dextrometorphan or other NMDA receptor antagonists has reduced postoperative pain, analgesic consumption or both and even beyond the clinical duration of action of the drugs (2-24) .
An effective premedicant minimizes the emotional trauma experienced by children when facing a surgery and it may facilitate a smoother anaesthesia induction with fewer complications. Preanesthetic medication with benzodiazepines reduces anxiety, produces sedation and anterograde amnesia. Oral premedication with midazolam is a common practice in the paediatric anaesthesia (22-24).
The aim of this study was to test the hypothesis that dextromethorphan, having sedative effects, can be used for premedication in children instead of midazolam. Additionally, becouse of its preemptive analgesic properties, we presumed a decreased level of postoperative pain and less postoperative analgesic consumption with dextromethorphan used for premedication.
MATERIALS AND METHODS:
After the Institutional Ethics committee's approval and informed parental consent have been received 70 ASA physical status I and II children were randomized by table of random numbers in two groups in a double-blinded manner. The first group (n=35) received oral dextrometorphan 0.5 mgkg -1 , and the second group (n=35) received oral midazolam 0.5 mgkg -1 45 min before the operation. Scheduled operations were hypospadias and hernia repair, circumcision or orchidopexy.
Age limit for the study was set at 5 years, for the ability to express VAS and faces score in younger children was questionable. Exclusion criteria were ASA III or more, any concomitant medical condition characterized with pain that could interfere with VAS scores, secondary infection, overweight children, inability to cooperate and parents' disapproval.
Induction of anaesthesia was with 8% sevoflurane and 50% nitrous oxide in oxygen, it was maintained with sevoflurane 2% end-tidal by face or laryngeal masks and spontaneous ventilation. All patients received diclofenac 1 mkg -1 rectally at the beginning of the operation what is the standard of care in our institution. Vital signs were measured: heart and respiratory rate, non-invasive blood pressure, end-tidal CO 2 by capnograph and SaO 2 by pulse oximeter. Intraoperative intravenous fluid therapy was 5 mLkg -1 h -1 of isotonic saline. Preoperative sedation was measured before the mask induction of anaesthesia by anaesthesiologist blinded to the premedication type. The postoperative evaluation of pain and sedation was performed by an independent anaesthesiologist.
We have measured analgesic requirements on the ward. Diclofenac suppository 1 mgkg -1 was administered as rescue analgesic when asked by the patient or when the visual analogue scale (VAS) exceeded 3. The total doses of diclofenac were also recorded. The side effects associated with dextromethorphan were recorded, if present.
University of Michigan Sedation Scale ( UMSS) scores were measured (0-awake/alert, 1-minimally sedated, tired, sleepy, appropriate response to verbal conversation or sounds, 2-moderately sedated ,somnolent, sleepy, easily aroused with light tactile stimulation, 3-deeply sedated ,deep sleep, arousable only with significant physical stimulation, 4 - unarousable) (29) at the induction of anaesthesia and two hours after the operation. The intensity of postoperative pain was evaluated using a visual analogue scale (VAS) consisting of a 10 cm line graded from 1 to 10 (1 =“no pain”, 10 = “the worst possible pain”) and faces pain scale at 2, 6 and 12 hours postoperatively ( FIGURE 1).
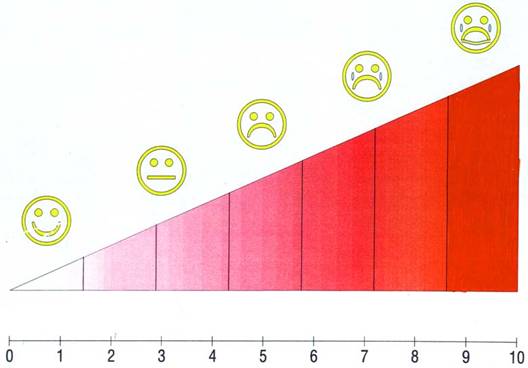 |
FIGURE 1. Visual analogue-scale (VAS) (1= “no pain”, 10= “the worst possible pain”) and Faces scale
In the statistical analysis the SPSS software version 11.0.1 was used. All data were presented as mean ± SD or Median (range) depending on the variable type and distribution. Since most variables were not normally distributed, only the non-parametric statistics have been done. For the ordinal variables (VAS, sedation scores) statistical significance was tested by chi-square test, while for numeric variables (number of rescue analgesia given) the significance was assessed by use of Mann-Whitney test for independent samples. P value less than 0.05 was considered statistically significant.
RESULTS
There was no statistically significant difference among the groups in terms of age, weight, gender, ASA physical status, the type and duration of operations (TABLE 1).
According to the sedation score, preoperative sedation was less with dextromethorphan, but didn't reach statistical significance (P=0.531) while postoperative level of sedation was significantly higher in the midazolam group (P <0.002) (FIGURE 2).
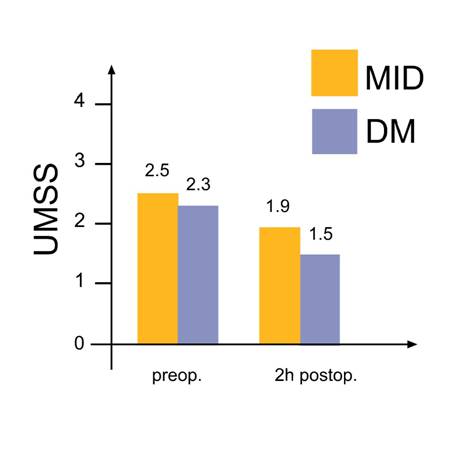 |
FIGURE 2. University of Michigan Sedation Scale (UMSS) before the operation and two hours postoperatively. DM-dextromethorphan; MID - midazolam.
Data are mean ± SD (MID 2.5±0.7 preoperatively, 1.9±0.7 postoperatively, DM 2.3±0.7 preoperatively, 1.5±0.5 postoperatively) p<0,002
Comparing the VAS between the two groups at 2, 6 and 12 hours postoperatively we have found that the difference at 2 and 6 hours was insignificant (P =0.635 and P =0.447) while the VAS scores at 12 hours were significantly lower in the dextrometorphan group (P <0.002) (FIGURE 3).
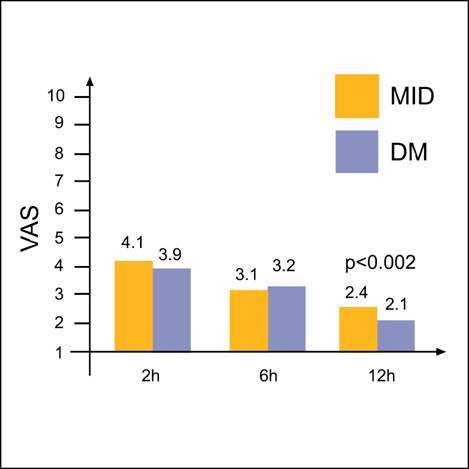 |
FIGURE 3. Visual-analogue (VAS) pain scores at 2,6 and 12 hours postoperatively.
DM-dextromethorphan, MID-midazolam. Data are mean ±SD ( DM at 2h 3.9±0.5,at 6h 3.2±0.8,at 12h 2.1±0.9;MID at 2h 4.1± 0.4 ,at 6h 3.1±0.9,at 12h 2.4±0.6) p<0,002
VAS scores in midazolam group were 2.4 and in the dextromethorphan group 2.1; both usually indicate the intensity of pain which requires no intervention and is therefore clinically not relevant.
The average number of rescue analgesics (diclofenac supp) did not show any significant difference between the two groups as determined by chi-square test (0.11 in the dextromethorphan group and 0.13 in the midazolam group ; P =0.451). There were no patients showing any adverse effects associated with the dextromethorphan.
DISCUSSION
Our study shows that in children dextromethorphan 0.5 mgkg -1 per os for premedication provides less preoperative sedation compared to midazolam. Because it does not relieve anxiety and has no anterograde amnestic effects as midazolam it is not useful for premedication. Sedation with midazolam was more effective after the operation what has clinical importance in one day surgery, implying possible prolonged discharge time.
In this randomized prospective study we have not found any significant differences in the quality of postoperative analgesia using two types of premedication : midazolam or dextromethorphan per os.
VAS scores at 2 and 6 hours postoperatively were not statistically significantly different and the need for rescue analgesia was the same in both groups. The only difference was found 12 hours postoperatively, when VAS scores in dextromethorphan group were significantly lower (2.1 comparing to 2.4 in midazolam group), but this difference is clinically irrelevant. Both scores are too low for some clinical intervention. Our study did not prove the hypothesis that dextromethorphan 0.5 mgkg -1 per os used for premedication in children has pre-emptive effect and decreases the level of postoperative pain and the amount of analgesic consumption.
The possible reasons for these findings could be: the dose of dextromethorphan is too small. Per orally dextromethorphan has a concentration of 1 mg ml -1 so with the applied dose of 0.5 mg kg -1 the volume of gastric of gastric juices may be to large. Increasing the dose the side effects like fatigue, dizziness, tremor, constipation and rush are more common.
The other reason may be the route of dextromethorphan application. Our institution policy is per os route for preanesthetic medication of children. It is possible that with other routes (IV, IM) results would be different regarding the pre-emptive effect of dextromethorphan.
It is possible that the follow-up period was too short. The pre-emptive analgesic effect would be confirmed if pain, analgesic consumption or both were significantly reduced five half-times beyond the administration of NMDA antagonist (3).Bearing in mind the half-life of dextromethorphan is 2-4 hours, our follow-up period of 12 h has partially not exceeded the proposed period.
Dextromethorphan, the d-isomer of the codeine analogue levorphanol is a morphinan without affinity for opioid receptors, commonly used as an antitussive medication with wide margin of safety. It has a mild sedative effect (4-11). Ketamine and dextromethorphan are clinically available non-competitive NMDA receptor antagonists. Dextrometorphan does not have a direct antinociceptive effect and therefore analgesia is achieved by its antagonistic action on the NMDA receptor, blocking the central sensitization (8, 9). Pre-emptive analgesia means treating the postoperative pain by prevention of central sensitization's occurrence (1,2).
The intravenous administration of large doses of dextromethorphan leads to hypotension and tachycardia (13). The bioavailability and the onset of action of intramuscular route is the same as intravenous, with fewer side effects (9). The bioavailability of the oral drug is only 10%. The onset of action occurs in 15-30 min after its oral administration (4, 6, 9, and 12).
Dextromethorphan, as other NMDA antagonists, can alleviate pain by reducing central hypersensitivity, by reducing tolerance to opioid analgesia when administered with opioids and by having an additional analgesic action when combined with NSAIDs (2).
There are many previous studies on pre-emptive dextromethorphan and acute pain with sometimes contradictory results (5-21).In adults results are satisfactory. The study of Kawamata et others (5) showed a beneficial effect of oral premedication with dextromethorphan 45 mg for reducing postoperative pain after tonsillectomy during 7 days. The study of Helmy and Bali (9) shows that preincisional administration of intramuscular 120 mg of dextromethorphan, compared with the same postincisional dose and a control in adults undergoing upper abdominal surgery, provides pre-emptive analgesia, as shown by prolonged time to the first use of rescue analgesic. The intramuscular route allows using a larger dose sufficient to block central sensitization and the onset of action is as rapid as by the intravenous route, but with fewer side effects. Ilkjaer and others (10) investigated the effect of 60 or 120 mg of oral dextromethorphan on primary and secondary hyperalgesia in 24 healthy male volunteers and concluded that dextromethorphan 120 mg reduces the magnitude of secondary hyperalgesia in humans. Wu and co-workers (11) concluded that the preincisional dextromethorphan 40 mg intramuscularly offers a pre-emptive analgesic effect thus improving the postoperative pain management after laparoscopic cholecystectomy in adults. In their previous study (12) they found that intramuscular dextromethorphan pre-treatment before surgical incision provided better postoperative pain relief during the first 72 hours following the upper abdominal surgery. Chia and colleagues (13) compared the preoperative versus postoperative administration of intravenous dextromethorphan 5 mgkg -1 in female patients undergoing lower abdominal surgery, and found that peroperative administration lowers morphine consumption for two days postoperatively, thus providing clinical evidence for pre-emptive effect of dextromethorphan.
Henderson and others investigated the effect of oral dextromethorphan (40 mg) on pain after hysterectomy, and found that over the next 48h the oral analgesic usage was significantly reduced (15). Weinbrom AA in many studies (16-21) demonstrated the positive effect of DM on immediate (2-6h) and late (24h to 3 days) postoperative analgesic requirements in cancer patients with lower body orthopaedic procedures. The failure of some studies to demonstrate a pre-emptive analgesic effect of dextromethorphan may be attributed to the use of small doses, oral administration, improper timing in the acute pain cases and its use in the established neuropathic pain
In children results are more controversial. Rose and colleagues (22) investigated postoperative pain after adenotonsillectomy in children and showed that premedication with dextromethorphan 0.5 or 1 mgkg -1 per os does not improve postoperative analgesia in school-aged children. To the contrary, Dawson and others (23) have concluded that dextromethorphan in a single oral dose of 1 mgkg -1 given 30 min before adenotonsillectomy significantly reduces the requirements for postoperative analgesia in children. The observation period was only 6 hours.
Hasan and colleagues (24) also have found that oral dextromethorphan 1 mg/kg per os reduces perioperative analgesic requirements in children after tympanomastoid surgery. These two authors used placebo and dextromethorphan, in a smaller group of children (18 to 20) and have not measured preoperative sedation.
CONCLUSION
Our study shows that dextromethorphan in a dose of 0.5 mgkg -1 per os can not be used for premedication in children, having mild sedative effects and no anxiolysis and anterograde amnesia, as midazolam has. We have found that postoperatively midazolam produces more sedation, what is not useful for one-day surgery.
With regard to the dextromethorphan's pre-emptive analgesic effects, the level of postoperative pain and the amount of postoperative analgesia requirements seemed equal between the groups in the early postoperative period (2 and 6 hours) and lower in the dextromethorphan group at 12h postoperatively, assuming possible pre-emptive effect of dextromethorphan on the late postoperative analgesia. Meanwhile, VAS scores in both groups of patients were to low for clinical interventions, so our hypothesis that dextromethorphan has pre-emptive analgesic properties wasn't confirmed.
REFERENCES
1. Woolf CJ, Chong MS. Pre-emptive analgesia–treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg 1993; 77:362-79.
2. Ong CKS, Lirk P, Seymour EA, Jenkins BJ. The efficacy of pre-emptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg 2005; 100:757-73.
3. Mc Cartney CJL, Sinha A, Katz J. A qualitative systematic review of the role of N-methyl-D-aspartate receptor antagonists in preventive analgesia. Anesth Analg 2004; 98:1385-400.
4. Petrenko AB, Yamakura T, Baba H, Shimoji K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: a review. Anesth Analg 2003; 97.1108-16.
5. Kawamata T, Omote K, Kawamata M, and Namiki A. Premedication with oral dextromethorphan reduces postoperative pain after tonsillectomy. Anesth Analg 1998;86:594-97.
6. Helmy SAK, Bali A. The effect of pre-emptive use of the NMDA receptor antagonist dextrometorphan on postoperative analgesic requirements. Anesth Analg 2001;92:739-44
10.Ilkjaer S,Dirks J,Brennum J,Wernberg M, Dahl JB. Effect of systemic NMDA receptor antagonist (dextromethorphan) on me and II hyperalgesia in humans. Br J Anaesth 1997;79.600-5.
11.Wu CT,Yu JC,Yeh CC , Liu ST et al. Preincisional dextromethorphan treatment decreases postoperative pain and opioid requirement after laparoscopic cholecystectomy. Anesth Analg 1999, 98:1331-4.
12. Wu CT,Yu JC, Liu St,Yeh CC, Li CY, Wong CS. Preincisional dextromethorphan treatment for postoperative pain management after upper abdominal surgery. World J Surg 2000; 24(5):512-7.
13. Chia YY, Liu K, Chow LH, Lee TY. The preoperative administration of intravenous dextromethorphan reduces postoperative morphine consumption. Anesth Analg 1999, 89:748-52.
14. Wadhwa A,Clarke D,Goodchild CS,Young D. Large-dose oral dextromethorphan as an adjunct to patient-controlled analgesia with morphine after knee surger. Anesth Analg 2001; 92(2):448-54.
15. Henderson DJ, Withington BS, Wilson JA, Morrison LM. Perioperative dextromethorphan reduces postoperative pain after hysterectomy. Anesth Analg 1999;89(2):399-402.
16.Weinbroum AA. Dextromethorphan reduces immediate and late postoperative analgesic requirements and improves patients' subjective scorings after epidural lidocaine and general anaesthesia. Anesth Analg 2002; 94(6):1547-52
17. Weinbroum AA,Gorodetzky A, Niv D,Ben-Abraham R,Rudick V, Szold A. Dextromethorphan attenuation of postoperative pain and primary and secondary thermal hyperalgesia. Can J Anaesth 2001; 48(2):167-74.
18. Weinbroum AA, Gorodetzky A et al. Dextromethorphan for the reduction of immediate and late postoperative pain and morphine consumption in orthopaedic oncology patients: a randomized, placebo-controlled, double-blind study. Cancer 2002; 95(5):1164-70.
19. Weinbroum AA, Bender B et al. Preoperative and postoperative dextromethorphan provides sustained reduction in postoperative pain and patient-controlled epidural analgesia requirements: a randomized, placebo-controlled, double-blind study in lower-body bone malignancy-operated pain. Cancer 2003; 97(9) 2334-40.
20. Weinbroum AA, Bender B et al. Dextromethorphan-associated epidural patient-controlled analgesia provides better pain- and analgesics-sparing effects than dextromethorphan-associated intravenous patient-controlled analgesia after bone-malignancy resection: a randomized, placebo-controlled, double-blinded study. Anesth Analg 2004;98(3).714-22.
21. Weinbroum AA,Lalayev G et al. Combined pre-incisional oral dextromethorphan and epidural lidocaine for postoperative pain reduction and morphine sparing: a randomized double-blind study on day-surgery patients. Anaesthesia 2001; 56(7):616-22.
22.Rose JB, Cuy R, Cohen De,Schreiner MS. Preoperative oral dextrometorphan does not reduce pain or analgesic consumption in children after adenotonsillectomy. Anesth Analg 1999; 88:749-53.
23. Dawson GS, Seidman P, Ramadan HH. Improved postoperative pain control in paediatric adenotonsillectomy with dextromethorphan. Laryngoscope 2001; 111:1223-6.
24. Hasan RA, Kortush JM, Thomas JD, Sigler DL. Oral dextromethorphan reduces perioperative analgesic administration in children undergoing tympanomastoid surgery. Otolaryngol Head Neck Surg 2004; 131(5):71-6.
25. Pywell CA, Hung YJ, Nagelhout J. Oral midazolam versus meperidine, atropine and diazepam: comparison of premedicants in paediatric patients. AANAJ 1995;63(2):124-30.
26.Fazi l,Jantzen EC et al. A comparison of oral clonidine and oral midazolam as pre-anaesthetic medications in the paediatric tonsillectomy patient. Anesth Analg 2001;92(1):56-61.
27. Bergendahl HT,Lonnquist PA,Eksborg S et al. Clonidine vs. midazolam as premedication in children undergoing adeno-tonsillectomy: a prospective , randomized,controlled clinical trial Acta Anaesthesiol Scand 2004;48:1292-300.
28. Viitanen H,Annila P, Viitanen M,Yli-Hankala A. Midazolam premedication delays recovery from propofol- induced sevoflurane anaesthesia in children 1-3 yr. Can J Anaesth 1999; 46:766-71.
29. Malviya S, Voepel-Lewis T, Tait AR ,Merkel S,Temper K,Naughton N. Depth of sedation in children undergoing computed tomography :validity and reliability of the University of Michigan Sedation Scale ( UMSS) Br J Anaesth 2'002;88:241-5.